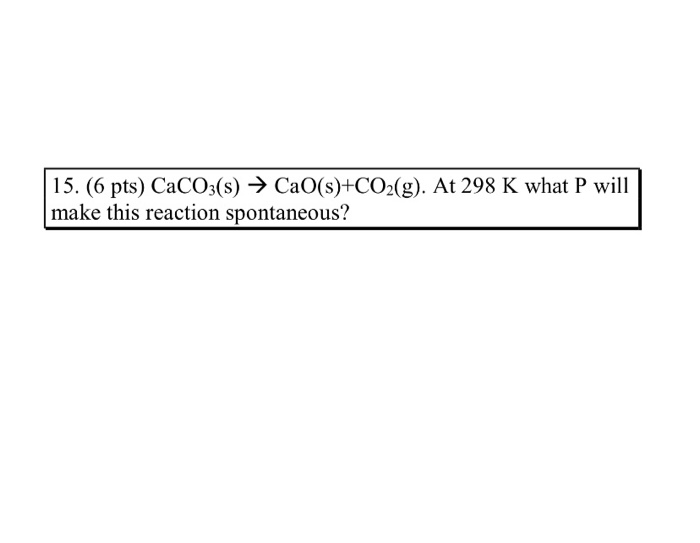
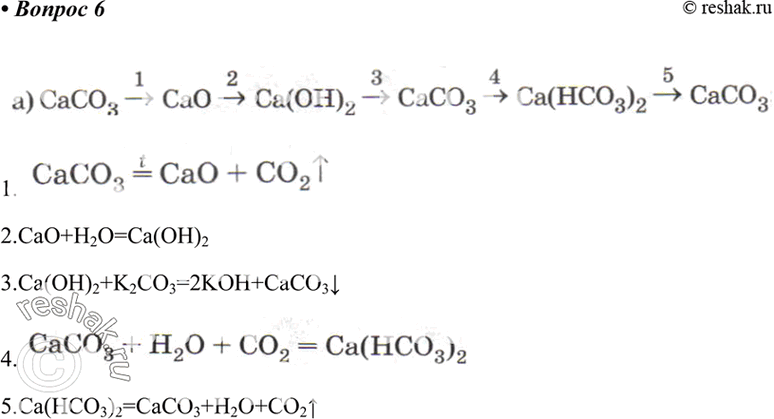In the equilibrium CaCO3(s)Cao (s)+CO2(g) at 1073 K, the pressure of CO2 isfound to be 2.5 x 10 Pa. The equilibriumconstant for the reaction at 1073 K will be.(a) 0.25(c)25(b) 2.5(d) 250
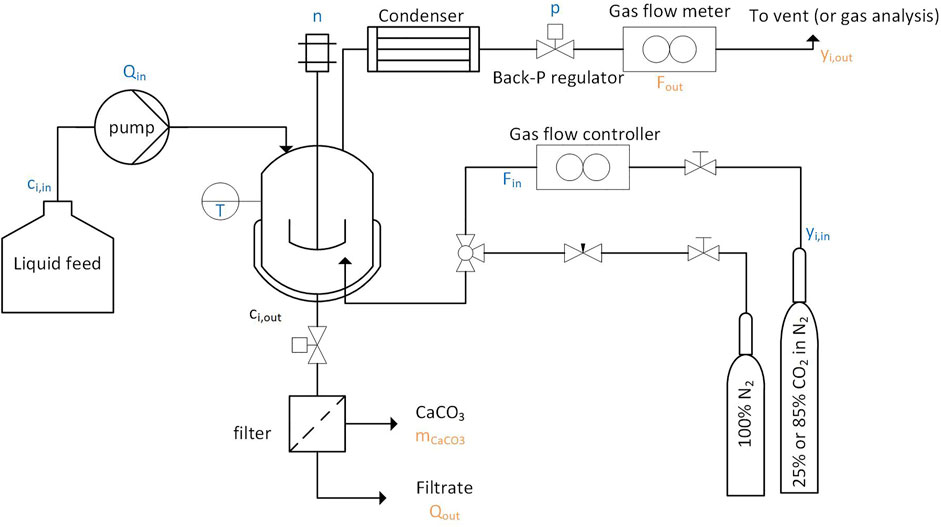
Frontiers | Experimental Investigation of a Continuous Reactor for CO2 Capture and CaCO3 Precipitation

Question Video: Writing a Net Ionic Equation for the Reaction of Solid Calcium Carbonate with a Hydrochloric Acid Solution | Nagwa
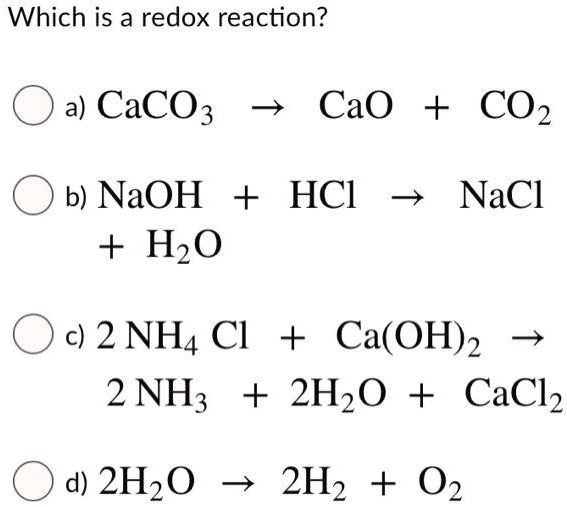
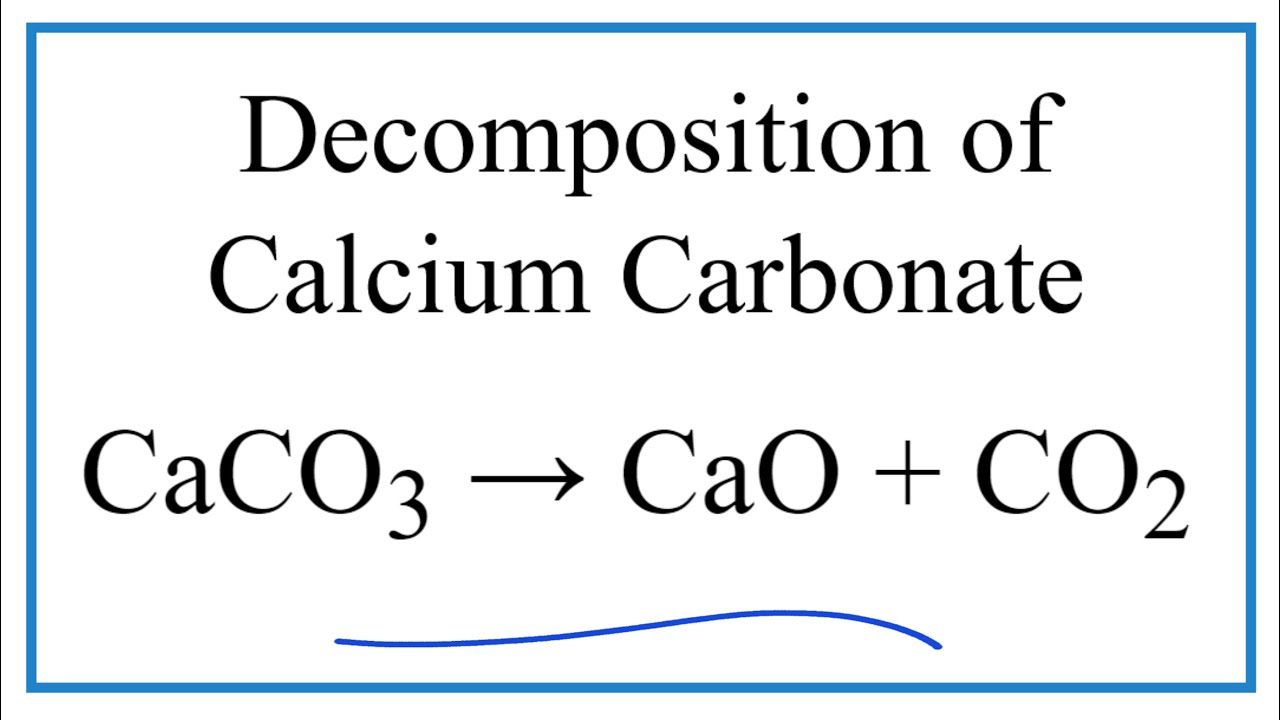
SOLVED: Which is a redox reaction? a) CaCO3 CaO + CO2 b) NaOH + HzO HCl NaCl c) 2 NH4 Cl + Ca(OH)2 2 NH; + 2Hz0 + CaClz d) 2Hz0 2H2 + 02
If 220 grams of calcium oxide (CaO) reacts with 50L of carbon dioxide (CO2), what mass of calcium carbonate (CaCO3) is produced? - Quora