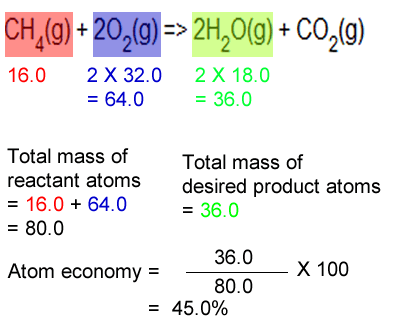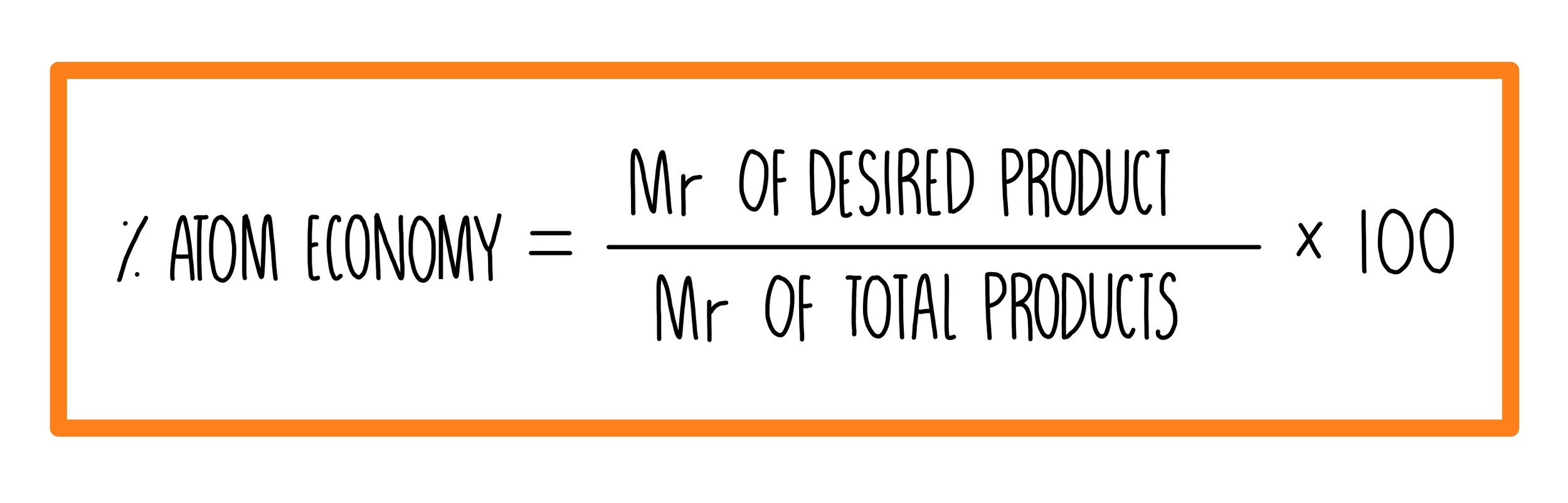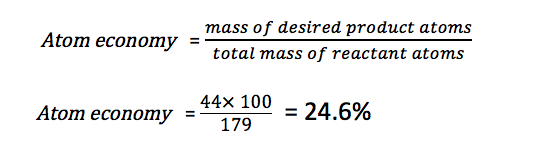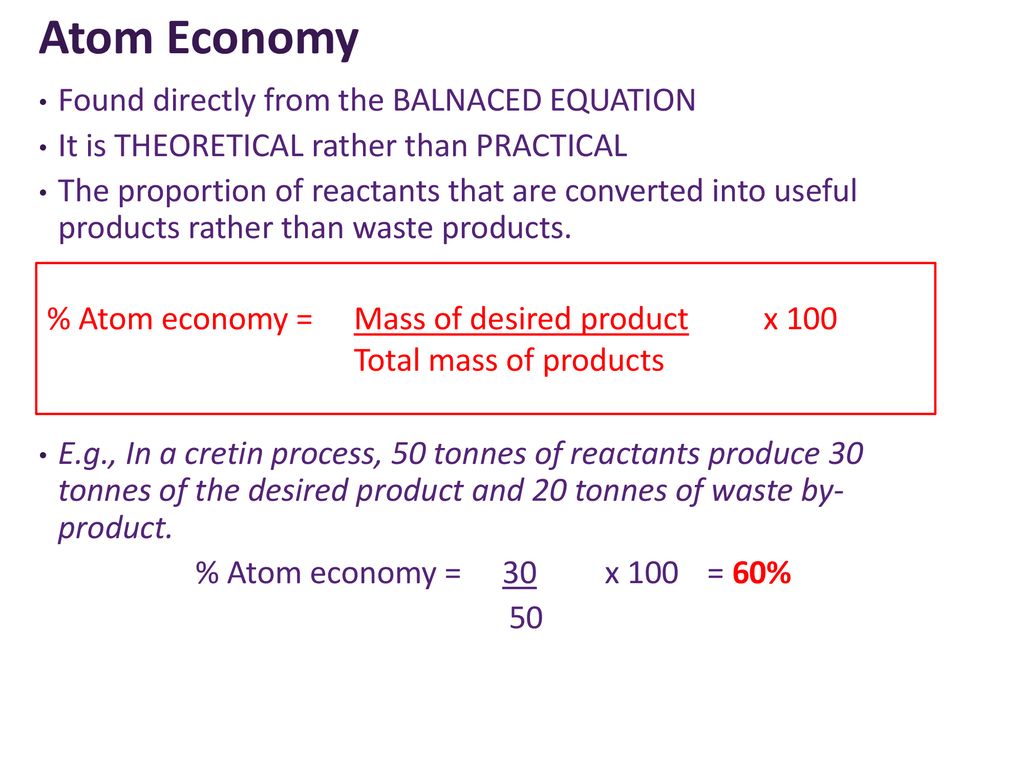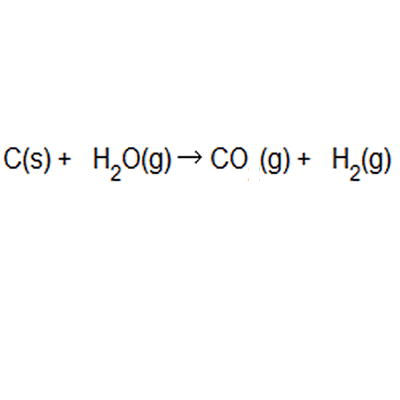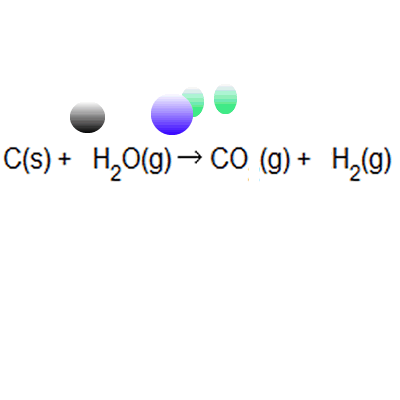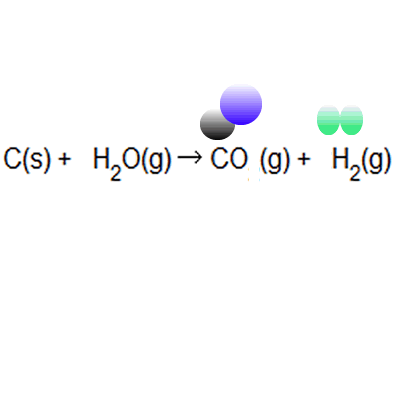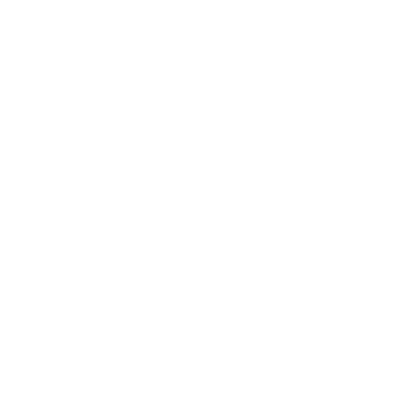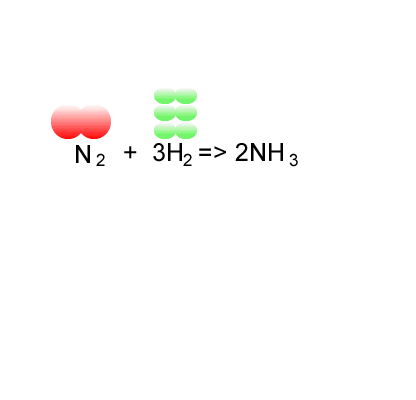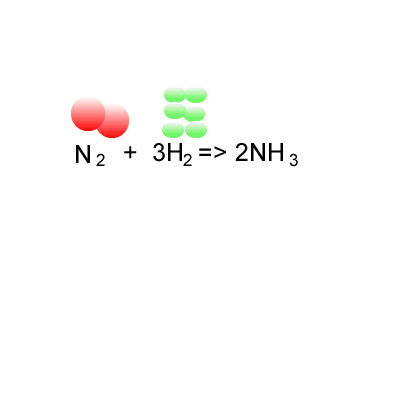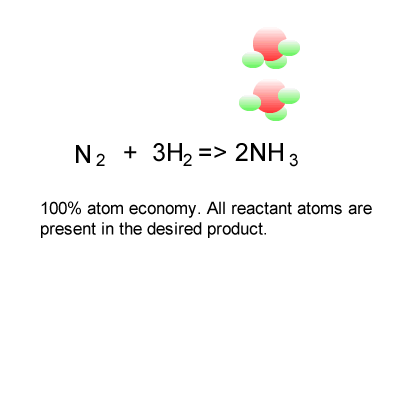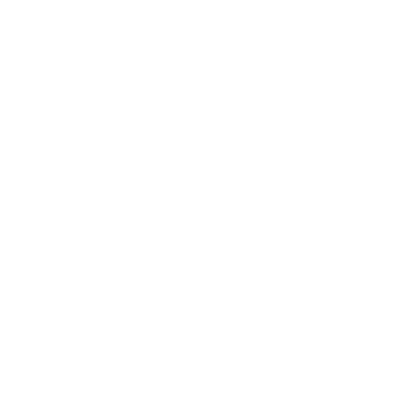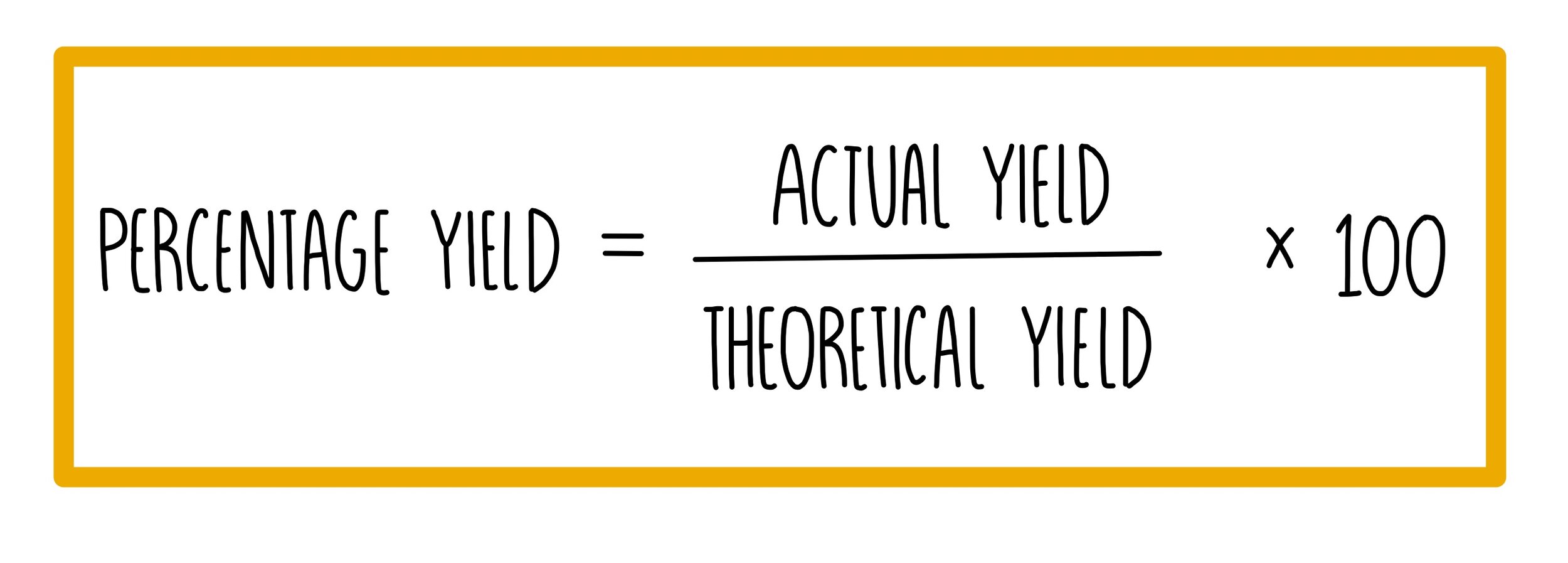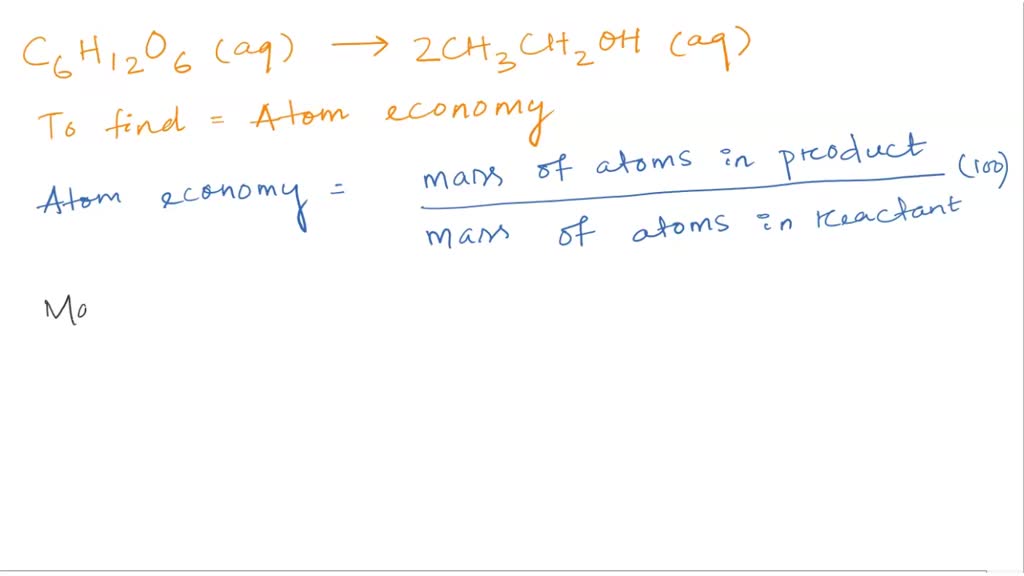
SOLVED: 56. Ethanol CH3CH2OH, can be produced by the fermentation of glucose, C6H12O6: C6H12O6 (aq) → 2CH3CH2OH(aq) The atom economy for the reaction is: Select one: 22.8 % 51.1% 35.3% 25.55% None of the suggested answers

molar gas volume Avogadro's Law moles and mass calculations gcse chemistry calculations igcse KS4 science A level GCE AS A2 O Level practice questions exercises

PDF) Molecular recycling: application of the Twelve Principles of Green Chemistry in the diversion of post-consumer poly(lactic acid) waste.