in a electrolytic tank aluminium metal is being extracted by d electrolysis of molten aluminium oxide using carbon electrodes.Ot is observed that one of the carbon electrodes is gradually burnt away and
A controllable membrane to pull carbon dioxide out of exhaust streams | MIT News | Massachusetts Institute of Technology

Aluminium oxide contains 52.9% aluminium and carbon dioxide contains 27.27% carbon. Assuming the....
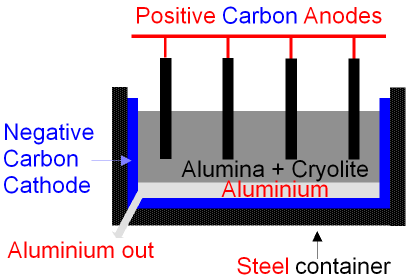
GCSE CHEMISTRY - Extraction of Aluminium - Electrolysis - Ionic Equations - Anode Replacement - GCSE SCIENCE.

a) Carbon concentrations in the bulk of a 100 nm-thick aluminum oxide... | Download Scientific Diagram
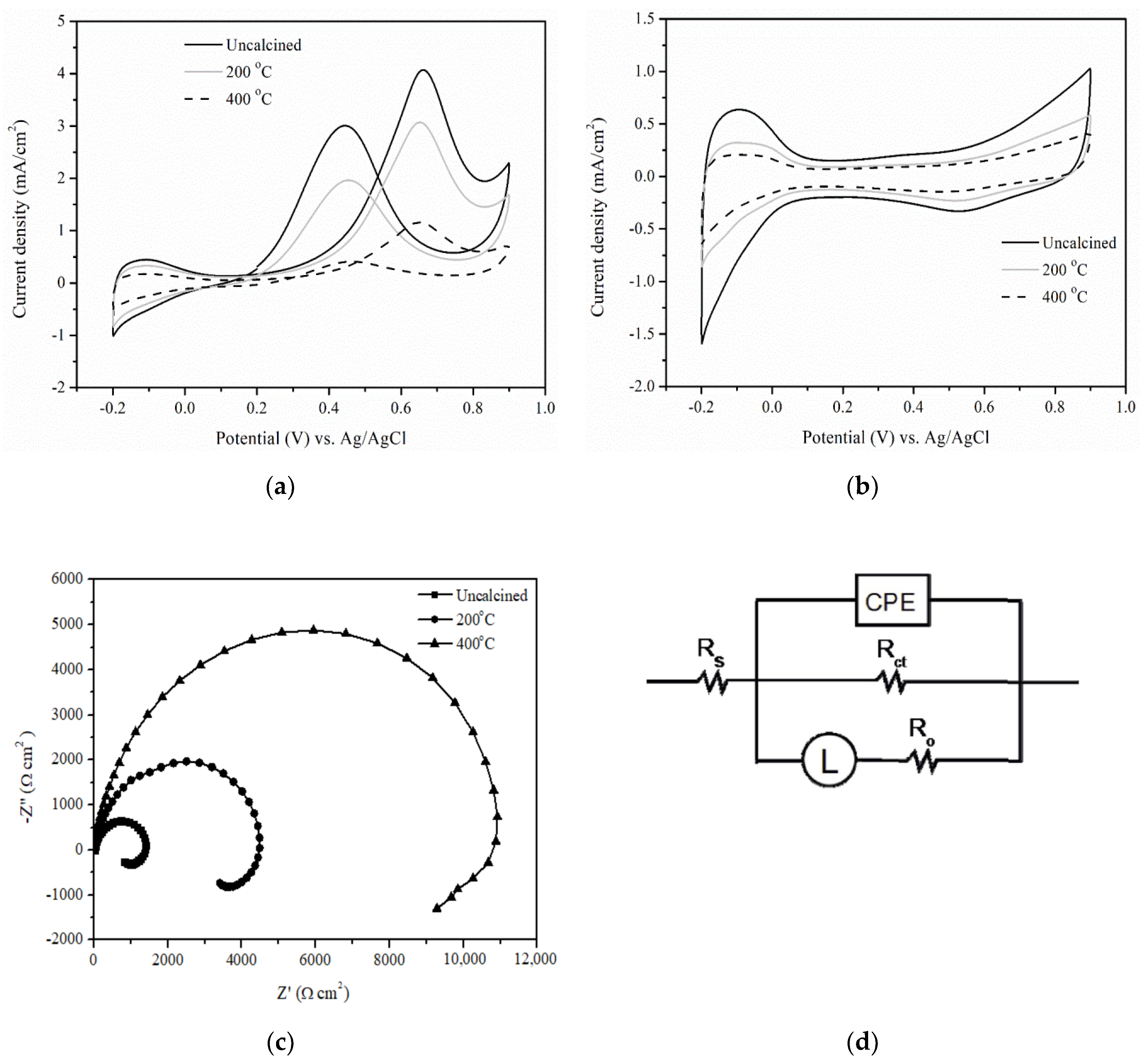
Catalysts | Free Full-Text | Pt-Amorphous Barium Aluminum Oxide/Carbon Catalysts for an Enhanced Methanol Electrooxidation Reaction


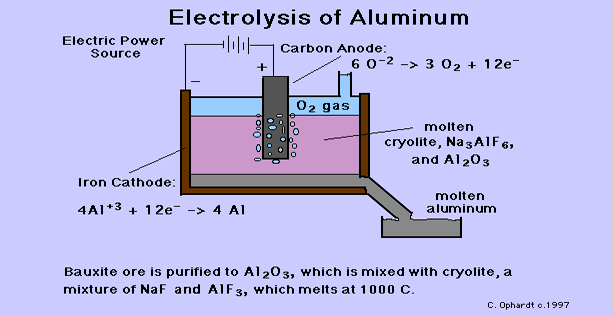


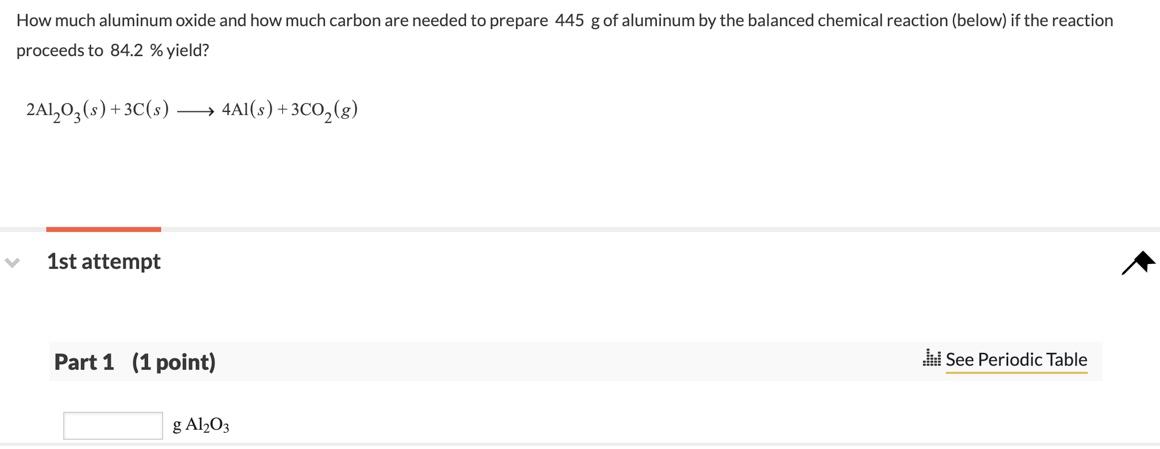

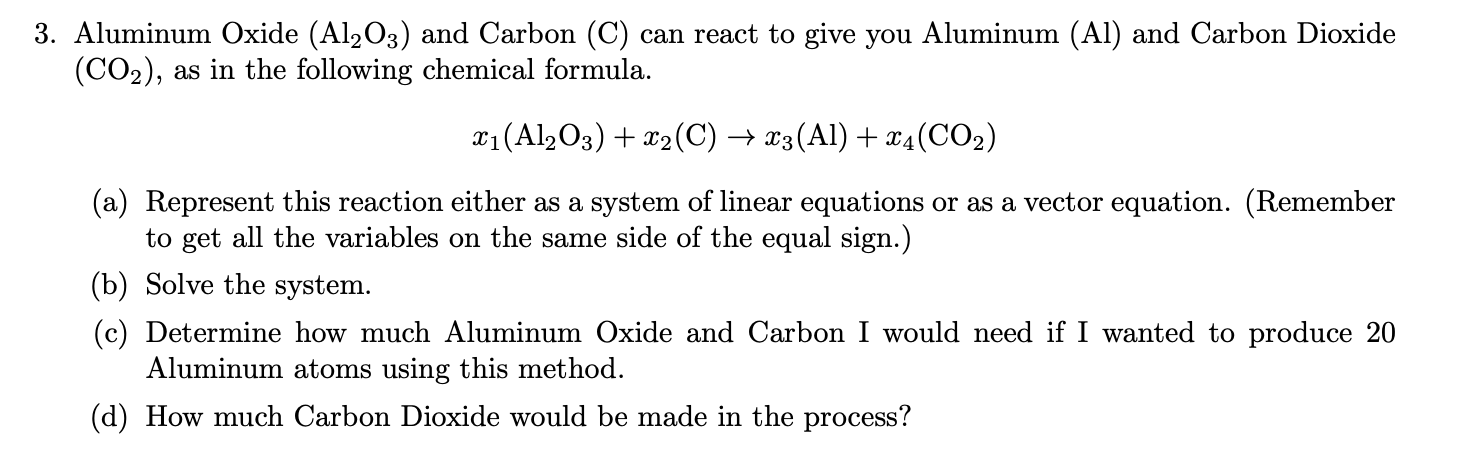
.png)